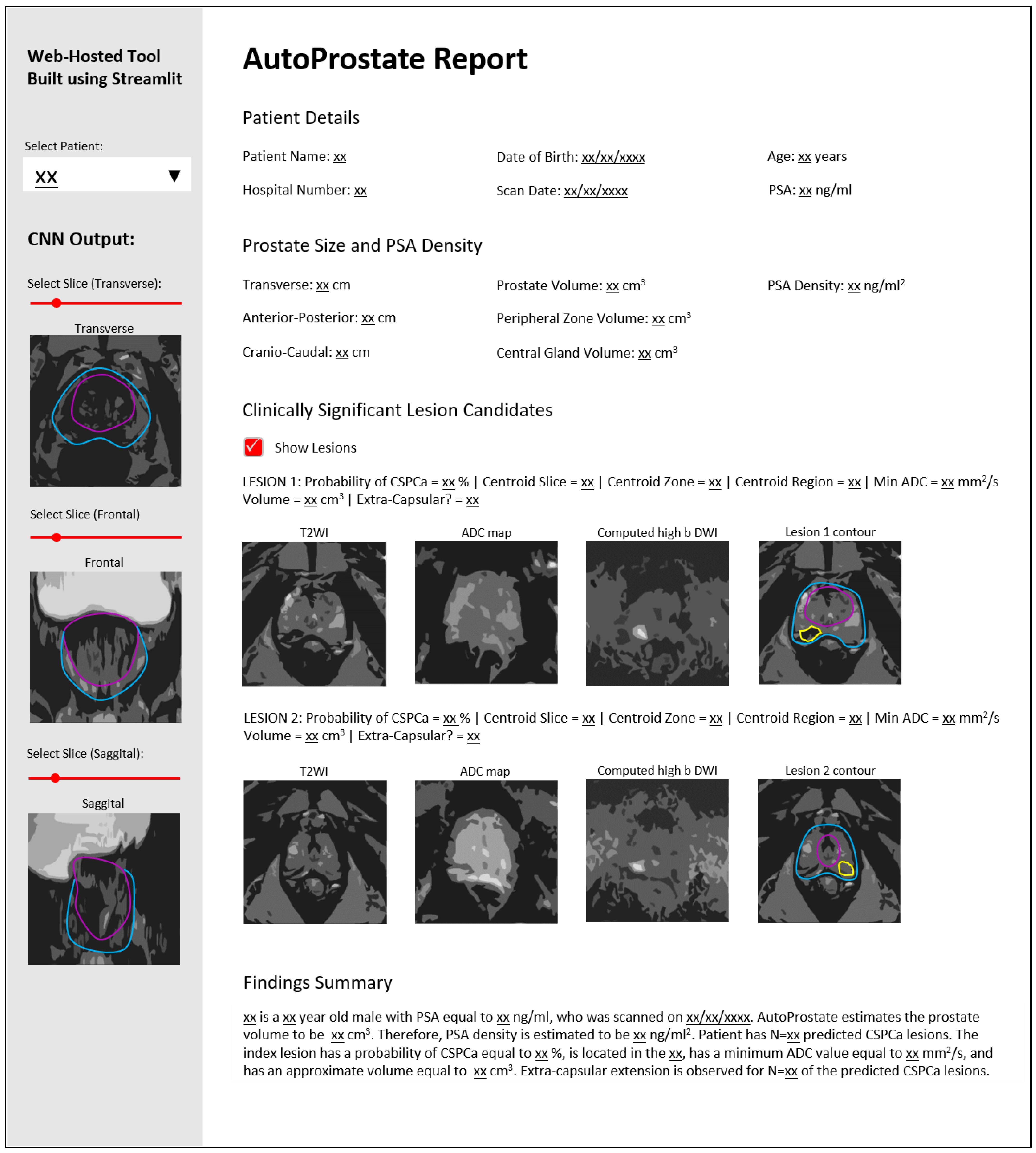
The Prostate MRI Reporting Template You Need to See: A Guide for Clinicians
Prostate MRI has revolutionized the diagnosis and management of prostate cancer. However, the interpretation and reporting of these complex images can be challenging. Standardized reporting is crucial for accurate communication between radiologists, urologists, and patients, ultimately impacting patient care. This article provides a comprehensive overview of a vital tool for this process: the Prostate MRI Reporting Template.
This isn’t just about filling out a form. It’s about ensuring clarity, consistency, and the highest quality of patient care. Let’s dive into what makes a good template and how it can benefit your practice.
Why a Prostate MRI Reporting Template is Essential
Before we look at the specifics, it’s important to understand why a standardized template is so valuable. Here’s a breakdown of the key benefits:
- Standardization: Ensures a consistent approach to image interpretation and reporting, regardless of the radiologist.
- Improved Communication: Facilitates clear and concise communication between radiologists, urologists, and other healthcare professionals.
- Enhanced Accuracy: Reduces the risk of overlooking critical findings and improves diagnostic accuracy.
- Facilitates Research: Allows for easier data collection and analysis, supporting research and advancements in prostate cancer management.
- Efficient Workflow: Streamlines the reporting process, saving time and reducing the potential for errors.
- Better Patient Management: Provides a clear and comprehensive picture of the patient’s condition, guiding treatment decisions and follow-up care.
Key Components of a Comprehensive Prostate MRI Reporting Template
A well-designed template should cover all essential aspects of the prostate MRI examination. Here’s what you should expect to find:
- Patient Demographics: Includes patient name, date of birth, MRN, and referring physician information.
- Clinical Indication: Clearly states the reason for the MRI (e.g., elevated PSA, abnormal DRE, prior biopsy).
- Technical Information: Details about the MRI protocol used, including:
- Sequence Types: T2-weighted, diffusion-weighted imaging (DWI), dynamic contrast-enhanced (DCE) imaging.
- Slice Thickness and Field of View (FOV).
- Contrast Agent Used (if applicable).
- Prostate Size and Shape: Measured and described.
- Zonal Anatomy Assessment: Detailed description of the peripheral zone (PZ), transition zone (TZ), and central gland. This includes:
- Assessment of Lesions: Using the Prostate Imaging Reporting & Data System (PI-RADS) version 2.1 classification.
- PI-RADS Score: Assigning a score (1-5) based on the likelihood of clinically significant cancer.
- Location of Lesions: Describing the anatomical location (e.g., apex, mid-gland, base; anterior, posterior, right, left).
- Size and Shape of Lesions: Measured in three dimensions.
- Characteristics of Lesions: Describing the features visualized on each sequence.
- Extracapsular Extension (ECE): Assessing for the presence of tumor extending beyond the prostate capsule.
- Seminal Vesicle Invasion (SVI): Assessing for tumor involvement of the seminal vesicles.
- Lymph Node Assessment: Evaluating regional lymph nodes for suspicious findings.
- Bone Assessment: Screening for bone metastases.
- Overall Impression and Recommendations: A concise summary of the findings, including:
- Overall PI-RADS Score: The highest PI-RADS score assigned.
- Recommendations for Further Management: Suggesting biopsy, active surveillance, or other appropriate follow-up.
Using the PI-RADS v2.1 Framework
The cornerstone of modern prostate MRI reporting is the Prostate Imaging Reporting & Data System (PI-RADS). The current version, PI-RADS v2.1, provides a standardized framework for:
Image Acquisition: Standardizing the MRI protocol across different institutions.
Image Interpretation: Providing clear guidelines for assessing various imaging features.
Risk Stratification: Assigning a PI-RADS score (1-5) to each lesion, reflecting the likelihood of clinically significant cancer:
- PI-RADS 1: Very low (clinically significant cancer is highly unlikely)
- PI-RADS 2: Low (clinically significant cancer is unlikely)
- PI-RADS 3: Intermediate (clinically significant cancer is possible; consider targeted biopsy)
- PI-RADS 4: High (clinically significant cancer is likely; consider targeted biopsy)
- PI-RADS 5: Very high (clinically significant cancer is highly likely; targeted biopsy is recommended)
Tips for Effective Prostate MRI Reporting
- Familiarize Yourself with PI-RADS v2.1: Understand the criteria for each PI-RADS score.
- Use a Structured Template: This ensures completeness and consistency.
- Be Precise and Specific: Avoid vague language. Use clear anatomical descriptions.
- Document All Findings: Even if benign, it’s crucial to record all observations.
- Correlate with Clinical Information: Consider the patient’s history, PSA levels, and other relevant data.
- Consider Multidisciplinary Collaboration: Discuss challenging cases with urologists and other specialists.
- Stay Updated: Keep abreast of the latest advancements in prostate MRI and reporting guidelines.
Conclusion: Empowering Better Patient Outcomes
A well-structured prostate MRI reporting template, built upon the foundation of PI-RADS v2.1, is an indispensable tool for radiologists and clinicians. It promotes accuracy, consistency, and clear communication, ultimately leading to more precise diagnoses and improved patient outcomes. By embracing these best practices, we can ensure that patients receive the highest quality of care in the fight against prostate cancer.
Frequently Asked Questions (FAQs)
- 1. Where can I find a good prostate MRI reporting template?
- Many radiology societies and professional organizations (e.g., the American College of Radiology) offer templates or examples. Your institution may also have its own standardized template. Seek resources from your network of medical professionals.
- 2. How often are PI-RADS guidelines updated?
- PI-RADS guidelines are periodically updated to reflect advancements in imaging technology and clinical understanding. PI-RADS v2.1 is the current version.
- 3. What is the role of DWI in prostate MRI?
- Diffusion-weighted imaging (DWI) is critical. It helps to visualize the movement of water molecules within tissues. In prostate MRI, restricted diffusion (appearing bright on DWI) is a key indicator of potential tumor presence.
- 4. What is the difference between a targeted biopsy and a systematic biopsy?
- Targeted biopsy is directed at areas of suspicion identified on MRI, based on the PI-RADS score. Systematic biopsy is a standard approach involving biopsies taken from multiple areas of the prostate, irrespective of the MRI findings.
- 5. How does the radiologist determine the PI-RADS score?
- The PI-RADS score is determined based on the characteristics of lesions visualized on different MRI sequences (T2-weighted, DWI, and DCE). The radiologist assesses each sequence and assigns a score based on the standardized criteria outlined in the PI-RADS guidelines.